 |
Home > R&D > Organizations |
|
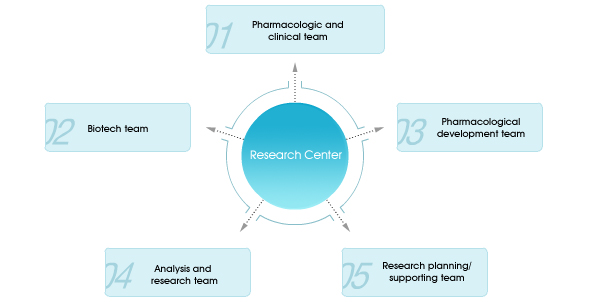
R&D center of Hyundai Pham is focusing on researching & manufacturing for new substances, and developing a variety of improved dosage. It also aims at research and development for expectorant, medicines for hypertension & dementia, and healthy food drinks. We are operating this expanded R&D center to foster efficiency of research and development with rapid decision and outstanding administration between employees. These efforts could be served as a foundation of improving an enormous capacity of R&D and innovative new drugs globally. Headquarter of development Since medicines & medical supplies are directly affecting human body, we should consider effectiveness as well as stability. For these reasons, it is possible to launch those products after getting an approval from KFDA. To get an approval of manufactured products, there is needed the result of clinical trials as well as consistent management in terms of its stability despite launching the products. The most important task for developing medicines is consistently launching new products and this process might be based on promoting the growth of a company in the future. To achieve these goals, we first need to construct product portfolios & vision for the company, and consistent management for success after developing competitive medicines according to the market needs. Developing medicines can be either development of new drugs/ IMDs/ generics through our own capacity of R&D, or co-licensing with globally well-know companies for business collaboration. Hyundai pharm is now making an effort to construct portfolios for hypertension, diabetes, hyperglycemia, and dementia for getting used to an aging society and promotion of national health. Planning office of clinical trials The planning office of clinical trials of Hyundai Pharm conducts clinical trials for an approval & registration from FDA, and establishes strategies for new products with a plan which aims at consistent growth domestically as well as globally. We are concretely not only focusing on the process of FDA and developing product lines whose total sales are over 20 % in the future, but also studying application of advanced technologies for promoting the products Hyundai pharm possesses. Hyundai pharm is now trying to be a believable pharmaceutical company through specialized and subdivided products. We are trying to not only conduct creative research and present theses, but also contribute medical growth through international collaboration. We will do our best to improve our research capacities though the innovative system respecting researchers’ abilities. Research center of healthy food The research center of healthy food has always challenged to help national health be improved as a leading company of healthy food drinks in Korea. As a result of this challenge, we launched ‘Miero fiber’ in 1989, Healthyoligo (made for intestine health) in 1990. Also, for the fist time among the companies of healthy food drinks, we successfully instituted ‘Non-GMO mark’, and launched ‘Miero beautyen’, drinking cosmetic drinks, to achieve our strategies that are developing innovative drinks. We will keep focusing on the safety of groceries on quality management we have stuck to since establishment, and also trying to conduct database regarding planning of patents and supporting academic things. Growth of the research center of healthy food will be continued. |
|
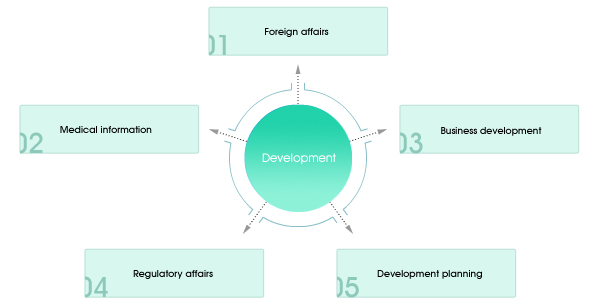
(Development : Mission) Development and sales of competitive new products to create profit and contribute to public health (Development : Strategies)
(Ways to Achieve Development Strategies)
|
||||||||||||||||||||||||||||||||||||
 |
 |
 |
 |
